
However, about 1 in 6 patients can develop breathing difficulties and in some of them the disease progresses to a more systemic disease and multiple organ dysfunction (1). Patient’s risk stratification is key to optimize COVID-19 patients flow at hospital. Stratification is currently based on characteristics of severe pneumonia and on laboratory findings.
Elevated D-dimer is now recognized to be associated with the severity of COVID-19 disease and is reported to be of poor prognosis (2). Table 1 shows D-dimer values in COVID-19 patients, from recent published papers; these observations notably show that markedly high D-dimer values are correlated with fatal outcome (2–5). Prolonged prothrombin time, decreased platelet count and lower fibrinogen levels are also commonly described in severe patients (2,3,6).
Altogether these findings would suggest that the development of active consumption coagulopathy, especially disseminated intravascular coagulation (DIC), is not rare in COVID-19 patients, and that its onset may unfavorably affect the clinical course.
Several international, national or local learning societies have issued recommendations for coagulopathy management in COVID-19 patients, such as ISTH (Internal Society of Thrombosis and Haemostasis), BSH (British Society of Haematology) or GFHT (French group for Haemostasis and Thrombosis)(7–11). All agree to recommend prophylactic anticoagulation, preferably with low molecular weight heparin (LMWH) provided patients do not suffer from severe renal impairment, even if dosage and patient’s selection may vary slightly between guidelines (12,13). It should however be considered to monitor LMWH with anti-Xa activity when LMWH dosage is superior than prophylactic regimen, or in renal impairment patients (10). In addition, all recent recommendations agree that a screening coagulation panel including D-dimer, PT, platelet count and fibrinogen may be of help to identify prospectively severe COVID-19 patients. These parameters may be used alone or within clinical scores such as ISTH-DIC score or SIC score (8,12).
It is worth noting that many unknowns remain in this infection. Guidelines authors thus indicate that their statements will be modified regularly according to developing knowledge.
Of interest is the recommendation on the use of VET methods in the management of bleeding in COVID-19 patients with clear safety warnings on their use mentioned in the ISTH interim guidance. This includes risk assessment showing that the system does not cause risk of aerosolisation of blood. For more information, please see: https://hemosonics.com/covid19/
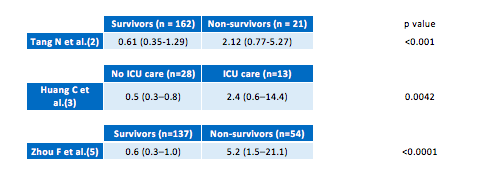
Table 1. D-dimer values from (2,3,5) in COVID-19 patients according to severity or clinical outcome.
Download the Covid-19 infection and thrombotic disorders paper.
